Fluorine is present in the Earth's crust at 544 ppm, making it the 13th most abundant element. Fluorine exists primarily in nature in the form of compounds such as fluorite (CaF2), calcium fluorophosphate (Ca10F2(PO4)6), and cryolite (Na3AlF6). Fluorine in rocks, minerals, and soil is the primary source of fluoride in surface and groundwater. The distribution of high-fluoride water in China is shown in Figure 1. Industrial production processes also emit large amounts of fluorine-containing waste gases, liquids, and residues. Fluorides that contribute to industrial fluorine pollution primarily come from aluminum and steel smelting in the metallurgical industry, phosphate fertilizer and fluoroplastic production in the chemical industry, brick, tile, ceramics, glass, and refractory production in the silicate industry, and coal-fired power generation in the power industry.
Waste gases and liquids directly pollute the environment, while fluorine-containing waste residues can also be an indirect source of fluoride pollution. These fluorine-containing waste gases, liquids, and residues are characterized by concentrated emissions, causing poisoning in nearby humans and livestock, and leading to endemic fluorosis.
Scientific research has found that fluoride has a strong affinity for calcium and phosphorus in the human body. It can disrupt the body's normal metabolism of calcium and phosphorus and inhibit the activity of certain enzymes, leading to a range of diseases including dental fluorosis, skeletal fluorosis, kidney, liver, and brain damage, immune dysfunction, pulmonary edema, pulmonary hemorrhage, and intellectual impairment in children.
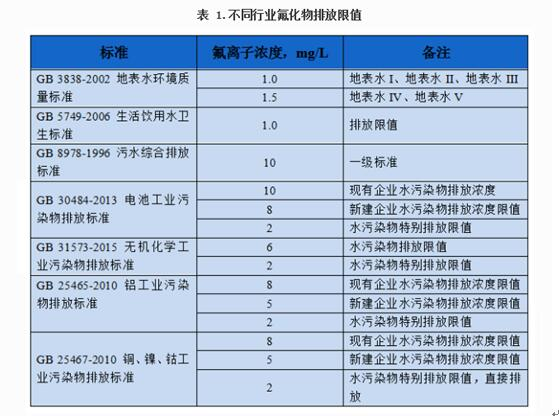
Fluoride exists in various forms in the natural environment. Fluoride primarily dissipates into the air as hydrogen fluoride (HF) and silicon tetrafluoride (SiF4). Fluoride in soil and water systems can be generally categorized as water-soluble, exchangeable, and adsorbed. Water-soluble fluoride primarily refers to fluoride present in soil and water solutions as ions or complexes, including F-, HF2-, H2F3-, H3F4-, and AlF63-. Organic ligands formed in water, such as those formed by humic substances, can also form complex complexes and chelates with fluoride and metal ions (such as Pb2+, Hg2+, Co2+, and Zn2+). The discharge standards for surface water, drinking water, sewage and fluorine-containing industrial wastewater are shown in Table 1.
There are many methods for treating fluoride-containing wastewater. Commonly used methods, both domestically and internationally, can be broadly categorized into two types: precipitation and adsorption. In addition to these two methods, there are also ion exchange resin defluorination, ultrafiltration membranes, electrocoagulation, and electrodialysis. However, due to high costs and low defluorination rates, these methods are rarely adopted as common defluorination processes.
Adsorption method: Typically, an adsorbent is loaded into a packed column and dynamic adsorption is used. This method is simple to operate and offers stable fluoride removal results, making it suitable for advanced drinking water treatment with smaller volumes. Common adsorbents used for fluoride removal include activated alumina, bone char, zeolite, bentonite, activated carbon, hydroxyapatite, and rare earth compounds such as zirconium oxide, which have high fluoride adsorption capacities. These adsorbents can reduce fluoride ion concentrations in wastewater to below 1 mg/L, meeting drinking water standards.
Precipitation methods
Precipitation methods mainly include chemical precipitation and coagulation-sedimentation.
For highly fluoridated water (fluoride ion concentration greater than 20 mg/L), lime or carbide slag precipitation is generally used. The calcium ions in the lime react with fluoride ions to form calcium fluoride precipitates, thereby removing fluoride ions. Chemical precipitation is characterized by its simple principle, convenient processing, low cost, and high effectiveness, and is currently widely used to treat highly fluoridated water. However, it has certain disadvantages, such as bulky equipment, difficulty meeting effluent standards after treatment, slow sedimentation, and difficulty in dewatering. Therefore, the addition of calcium chloride or other coagulants is often necessary to accelerate precipitation.
For low-fluoridated water (fluoride ion concentration less than 20 mg/L), coagulation-sedimentation is generally used. Coagulants form positively charged colloids in the water that absorb F-ions, causing the colloids to coalesce into larger flocs and precipitate, achieving fluoride removal. Coagulation-sedimentation offers advantages such as low reagent dosage and high water volume treatment. However, it is generally only suitable for treating water with low fluoride content. For better results, it must be combined with other methods. However, the defluoridation effect is greatly affected by operational factors such as stirring conditions and settling time, as well as the concentration of anions such as CO32-, SO42-, and Cl- in the water. In recent years, with the continuous development of the chemical and water treatment industries, many high-efficiency coagulant defluoridation agents have emerged, revitalizing enhanced coagulation technology. With the application of sensing and automatic control technologies, real-time control of coagulant dosage has become possible.
Organic fluorine wastewater treatment equipment

How to remove fluoride from water? Depending on the amount of water and the pollutants it contains, the equipment style and price are also different. For more details or to find a unit to handle it, please log on to the Wastewater Treasure website for inquiries. If you have fluoride-containing wastewater treatment needs, please consult Shandong Hongke Hydropower Equipment Co., Ltd