The discharge of laundry wastewater accounts for more than 20% of the total domestic sewage, making it a potential reusable water resource. Currently, domestic laundry wastewater is usually collected uniformly through urban sewage pipe networks and transported to sewage treatment plants for processing. However, laundry wastewater contains a large amount of surfactants. When the surfactant load exceeds a certain level, it will reduce microbial respiration, interrupt phosphorus absorption, affect the morphology of activated sludge, cause floc breakage and protozoan cell lysis, and thereby reduce the treatment efficiency of sewage treatment plants. If laundry wastewater can be treated and reused separately, it can not only reduce the load of sewage treatment plants but also improve the recycling rate of sewage and save water resources.
1.1 Raw Experimental Water
1.2 Experimental Equipment and Methods
1.2.1 Experimental Equipment
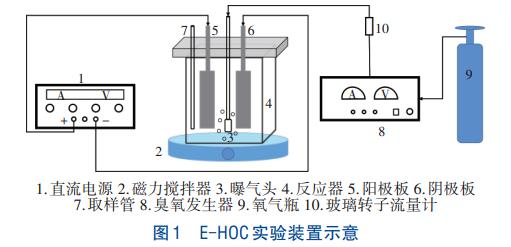
The experimental equipment is shown in Figure 1. A rectangular organic glass electrolytic reactor was used, with dimensions (length × width × height) of 10 cm × 7 cm × 12 cm, and the volume of water sample used in each experiment was 500 mL. The anode material was aluminum electrode or Dimensionally Stable Anode (DSA) electrode, and the cathode material was 316-grade stainless steel or carbon fiber plate. The electrodes were completely immersed in water, with an effective surface area of 84 cm² and dimensions (length × width × thickness) of 7 cm × 6 cm × 0.1 cm, and the distance between the plates was 2 cm. Before the experiment, the electrodes were first polished with sandpaper, then cleaned with 1 mol/L HCl solution for 10 minutes, rinsed with deionized water, and finally dried for use. The electrodes were directly connected to a regulated DC power supply. During the reaction, a magnetic stirrer was used for stirring at a speed of 100 r/min. An oxygen-source ozone generator was used to produce ozone, and the ozone flow rate was controlled by a gas flowmeter.
1.2.2 Experimental Methods
Take 500 mL of the experimental water sample, add an appropriate amount of electrolyte Na₂SO₄ to the water sample according to the current density, and stir thoroughly with a magnetic stirrer to completely dissolve Na₂SO₄. Before the experiment, set the current density (5, 10, 15, 20 mA/cm²) and ozone flow rate (100, 200, 300, 400 mL/min). Connect the electrodes to a DC power supply. In the coupled system, connect the ozone generator to an aeration head, and turn on the ozone generator and DC power supply simultaneously. At this point, the EC and O₃ oxidation reactions occur simultaneously, with a reaction time of 1 hour. In the non-coupled system, the EC stage has a reaction time of 1 hour, and the subsequent stage has a reaction time of 1 hour, with a total reaction time of 2 hours. After the reaction, let it stand for sedimentation for 30 minutes, then take samples to determine Chemical Oxygen Demand (COD), turbidity, Linear Alkylbenzene Sulfonate (LAS), etc. For kinetic analysis, samples were taken every 10 minutes.
1.3 Analysis Items and Methods
COD: Rapid digestion spectrophotometry.
Turbidity: Portable turbidimeter.
LAS: Methylene blue spectrophotometry. Before determination, filter the water sample with medium-speed qualitative filter paper first.
Microplastics: Refer to the method of Tian et al. Collect three batches of drainage during household laundry (washing wastewater, first rinsing wastewater, second rinsing wastewater), mix and stir evenly, take 500 mL of the water sample, and filter it with a 0.7 μm glass fiber filter membrane. Then place the filter membrane in an evaporating dish, wrap it with aluminum foil, pierce several small holes on the surface of the aluminum foil with a needle, dry it in an oven at 60 °C for 1 hour, and then observe and count under a microscope.