Comparison of Laundry Wastewater Treatment Processes
Under the conditions of a current density of 10 mA/cm² and an ozone flow rate of 200 mL/min, the COD removal effects of twelve processes on laundry wastewater were compared, including the electrocoagulation process (EC), ozone oxidation process, electrocoagulation-magnetic separation coupling process, electrocoagulation-ozone oxidation coupling process (E-HOC), electrocoagulation combined with ozone catalytic oxidation process (EC+O₃/O₃ catalyst), electrocoagulation combined with electrocatalytic oxidation process (EC+EO), electrocoagulation-hydrogen peroxide coupling process (EC/H₂O₂), ultraviolet-hydrogen peroxide coupling process (UV/H₂O₂), ultraviolet-ozone oxidation coupling process (UV/O₃), electrocoagulation combined with ultraviolet-ozone oxidation process (EC+UV/O₃), ultraviolet-hydrogen peroxide-ozone oxidation coupling process (UV/H₂O₂/O₃), and iron-carbon-hydrogen peroxide coupling process. The results are shown in Figure 2 (where a~l on the horizontal axis of the figure represent the above twelve processes in sequence).
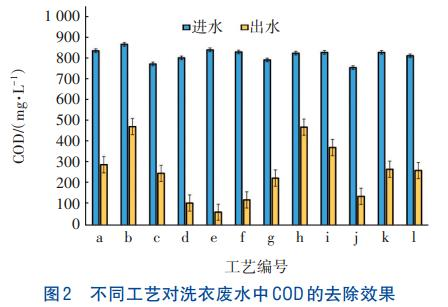
As can be seen from Figure 2, the processes with electrocoagulation (EC) as the core exhibit significantly better COD removal efficiency for laundry wastewater than other non-EC processes, so EC-based processes are prioritized. Under the same conditions, coupled process systems and EC-series oxidation processes achieve better COD removal effects compared to standalone EC and standalone oxidation processes, with the COD removal rate increased by 16.3%~47%. Compared with the EC process, the COD removal rates of E-HOC, EC+O₃/O₃ catalyst, EC+EO, and EC+UV/O₃ processes are increased by 21.3%, 27.2%, 20%, and 16.3% respectively. This may be due to the introduction of ozone and electrocatalysis increasing the generation of ·OH in the system, thereby promoting the removal of organic matter. Additionally, DSA electrodes have high electrocatalytic activity during electrolysis and can generate ·OH, which is consistent with the research results of Heffron et al. The ozone and ozone/catalyst systems produce ·OH, ·O₂⁻, and H₂O₂ during the reaction, and ·O₂⁻ is converted to ·OH in the system, further enhancing COD removal.
Compared with the standalone O₃ oxidation process, the COD removal rates of E-HOC, EC+O₃/O₃ catalyst, and EC+UV/O₃ processes are increased by 41.1%, 47%, and 36.1% respectively. EC is often used as a pretreatment process for removing turbidity and natural organic matter, generating coagulants in situ in water through sacrificial anodes. When carbon fiber is used as the cathode, H₂O₂ is also produced, thereby promoting the further removal of COD from laundry wastewater. Bernal-Martinez et al. found that the combined electrocoagulation-ozone process can achieve 60% removal rates of COD and BOD₅ in industrial wastewater, while the E-HOC process can achieve 84%, 79%, 95%, 96%, and 99% removal rates of COD, BOD₅, chroma, turbidity, and total coliforms respectively. Garcia-Morales et al. obtained similar results, showing that the synergistic effect of the coupled electrocoagulation-ozone process improves the removal effects of chroma, turbidity, and COD.
Therefore, the EC-based processes, including electrocoagulation-ozone oxidation coupling process (E-HOC), electrocoagulation combined with electrocatalytic oxidation process (EC+EO), electrocoagulation combined with ozone catalytic oxidation process (EC+O₃/O₃ catalyst), and electrocoagulation combined with ultraviolet-ozone oxidation process (EC+UV/O₃), are selected as the preferred processes for laundry wastewater treatment.
Analysis of Pollutant Removal Effects in Laundry Wastewater
1 Pollutant Removal Effects Under Different Current Densities
Controlling the ozone flow rate at 200 mL/min, the removal effects of five processes (SS-E-HOC, C-E-HOC, EC+O₃/O₃ catalyst, EC+UV/O₃, and EC+EO) on COD, turbidity, and LAS in laundry wastewater were investigated under the conditions of current densities of 5, 10, 15, and 20 mA/cm² in the electrocoagulation stage. The results are shown in Figure 3. It can be seen that when only the current density in the electrocoagulation stage is changed, the EC+UV/O₃ process has the best COD removal effect, with the highest COD removal rate reaching 89.8% at a current density of 10 mA/cm². Followed by the EC+O₃/O₃ catalyst and C-E-HOC processes. For turbidity, the SS-E-HOC and C-E-HOC processes have the best removal effects, with removal rates both above 90%. The three processes of EC+O₃/O₃ catalyst, EC+UV/O₃, and C-E-HOC have better LAS removal effects, with removal rates reaching over 98.2%.
In addition, in the EC+EO process, the current density in the electrocoagulation stage was controlled at 10 mA/cm², and the current density in the electrocatalytic stage was changed to 5, 10, 15, and 20 mA/cm² respectively to investigate the removal effects of the process on COD, turbidity, and LAS in laundry wastewater. The results show that under the four current densities, the COD removal rate of the EC+EO process is all below 80%. When the current density in the electrocatalytic stage is 10 mA/cm², the turbidity removal rate of the EC+EO process is the highest, exceeding 90%. When the current density in the electrocatalytic stage is 10, 15, and 20 mA/cm² respectively, the LAS removal rate of the EC+EO process all exceeds 90%.
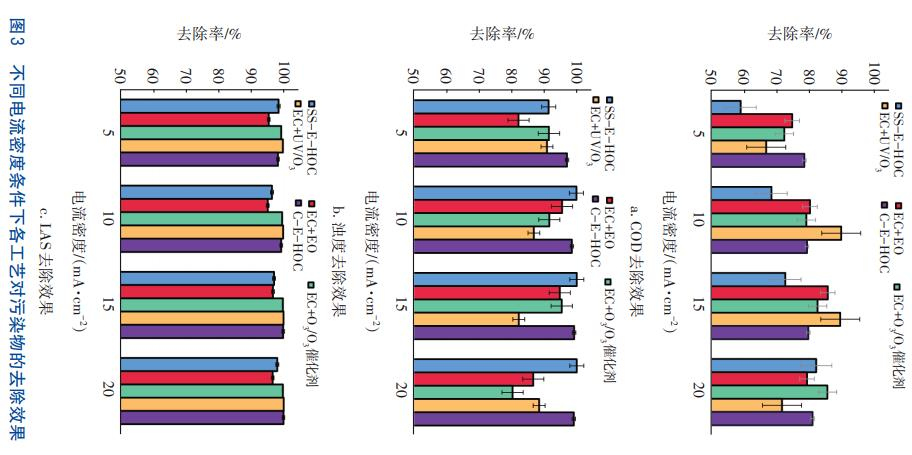
Pollutant Removal Effects Under Different Ozone Flow Rates
Controlling the current density in the electrocoagulation stage at 10 mA/cm², the removal effects of different processes on COD, turbidity, and LAS in laundry wastewater were investigated under the conditions of ozone flow rates of 100, 200, 300, and 400 mL/min respectively. The results are shown in Figure 4. It can be seen that the COD removal rates of the C-E-HOC and EC+UV/O₃ processes can reach over 90%, the maximum turbidity removal rates of the SS-EHOC and C-E-HOC processes reach 100%, and the LAS removal rates of the EC+O₃/O₃ catalyst, EC+UV/O₃, and C-E-HOC processes can reach over 99%. Although the COD removal rate of the EC+O₃/O₃ catalyst process can exceed 80%, the effluent quality fails to meet the reuse standard.
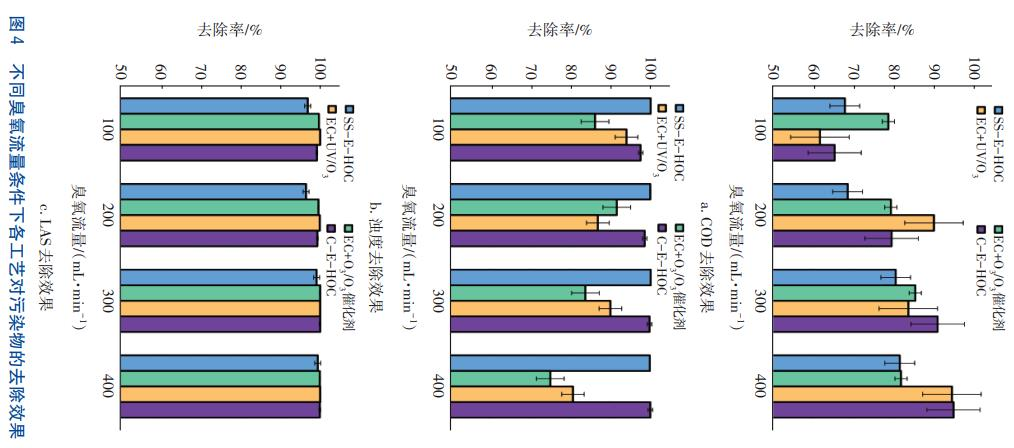
After comprehensive consideration, the electrocoagulation-ozone oxidation coupling process (E-HOC) is ultimately selected as the treatment process for laundry wastewater. Under the conditions of a current density of 15 mA/cm² and an ozone flow rate of 400 mL/min (corresponding to an ozone dosage of 69.12 mg/L), the removal rates of COD, turbidity, and LAS in laundry wastewater by this process are 97.6%, 99.9%, and 99.6% respectively. The effluent quality meets the standards specified in GB/T 18920—2020 (Water Quality Standard for Urban Reclaimed Water for Urban Miscellaneous Use) and GB/T 19923—2005 (Water Quality Standard for Urban Reclaimed Water for Industrial Use), satisfying the reuse requirements. In addition, the cathode of the process is a carbon fiber electrode plate. The concentration of H₂O₂ at 10 minutes of reaction is measured as 4.42 mg/L by the titanium salt spectrophotometric method, proving that H₂O₂ is generated during the reaction.
The treatment cost is estimated based on the ozone generator, DC power supply power, reaction time, etc., as shown in Equation (1). EEO is defined as the electrical energy (kW·h) required to reduce the pollutant concentration in 1 m³ of water by one order of magnitude, where U is the voltage (V), I is the applied current (A), T is the electrolysis time (h), V is the volume of water in the reactor (m³), and C₀ and C are the initial and final COD concentrations (mg/L) respectively. Under the optimal operating conditions, the energy consumption of the process is 86.2 kW·h/(m³·order), and this technology can be applied to the wastewater treatment of hospitals, hotels, and laundry factories.

Kinetic Analysis and Microplastic Removal Characteristics
1 Kinetic Analysis
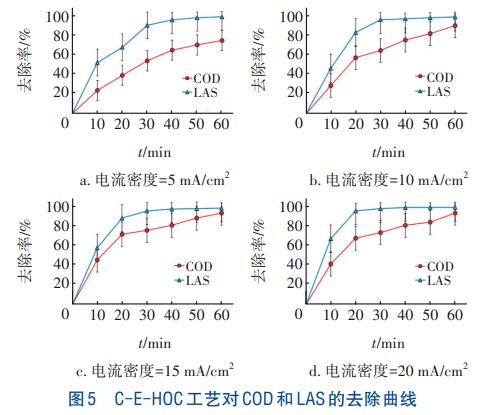
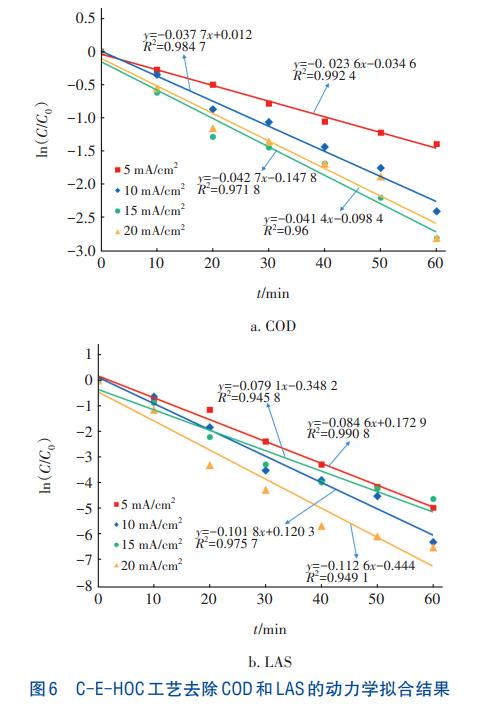
Based on the above results, kinetic analysis of COD and LAS removal from laundry wastewater by the C-EHOC process was conducted under the conditions of an ozone flow rate of 400 mL/min and current densities of 5, 10, 15, and 20 mA/cm². The total reaction time was 60 minutes, and samples were taken every 10 minutes. The removal curves of COD and LAS are shown in Figure 5. With the progress of the reaction, the removal rates of both COD and LAS increased rapidly in the first 30 minutes, while the increasing rate slowed down in the latter 30 minutes, and the removal curves became gentle. For COD, the removal rate in the first 30 minutes was the fastest at a current density of 15 mA/cm²; for LAS, the removal rate in the first 30 minutes was the fastest at a current density of 20 mA/cm². Fitting results indicated that the removal processes of COD and LAS by the C-EHOC process conformed to the first-order reaction kinetic model, as shown in Figure 6.
Microplastic Removal Characteristics
Microplastics generally refer to plastic fibers, fragments, and particles with a size of less than 5 mm. Microplastics released from synthetic textiles during the washing process are considered a major source of microplastics in oceans and wastewater treatment plants. In this study, it is estimated that (40~150) × 10⁴ microplastics are released into the environment per 100 L of water during domestic laundry. The C-E-HOC process can efficiently remove microplastics from laundry wastewater. Under the conditions of a current density of 15 mA/cm² and an ozone flow rate of 400 mL/min, the microplastic removal rate of the C-E-HOC process can reach 94.2%. It is inferred that the efficient removal of microplastics by the C-E-HOC process is mainly attributed to electrocoagulation. Aluminum anodes can in-situ generate aluminum salt coagulants during the reaction, which form flocculent precipitates to remove microplastics from water through mechanisms such as electrical neutralization, adsorption bridging, and net trapping